Absorbance Spectroscopy to Detect Differences in Sparkling Water Flavor
Background/Applications:
Each year, many people make New Year’s resolutions which are personal goals or commitments that a person makes for the New Year, often related to self-improvement. One of the most common resolutions is losing weight or generally having a healthier diet. To maintain eating healthier, many individuals will replace high-calorie soft drinks with sugar-free alternatives that use flavoring to mimic the sweetness of the substituted drink. While these lower-calorie drinks will sometimes have color dyes added to them, often they are simply clear and carbonated flavored drinks. This can make it difficult to discern between flavors, especially when different flavors may have similar color brandings associated with them, such as black cherry and blueberry pomegranate. If the flavors cannot be distinguished visually, perhaps other methods may be used to discern between flavors. This led to our experiment idea to use absorbance spectroscopy to detect subtle differences in the chemical composition of different flavored sparkling waters to see if we could distinguish flavor types.

Figure 1: Sparkling water samples used for the experiment
This experiment aims to differentiate between the flavor additives found in four different flavored sparkling waters, including lemon-lime, blueberry pomegranate, white grape, and black cherry by looking at the wavelength differences using an Avantes absorbance spectroscopy setup. Seltzer water will be used as a reference since it is a flavor-free carbonated beverage. As an additional comparison, we will also analyze a lemon-lime soda to assess the added flavor amount versus the lemon-lime sparkling water.
Description of System:
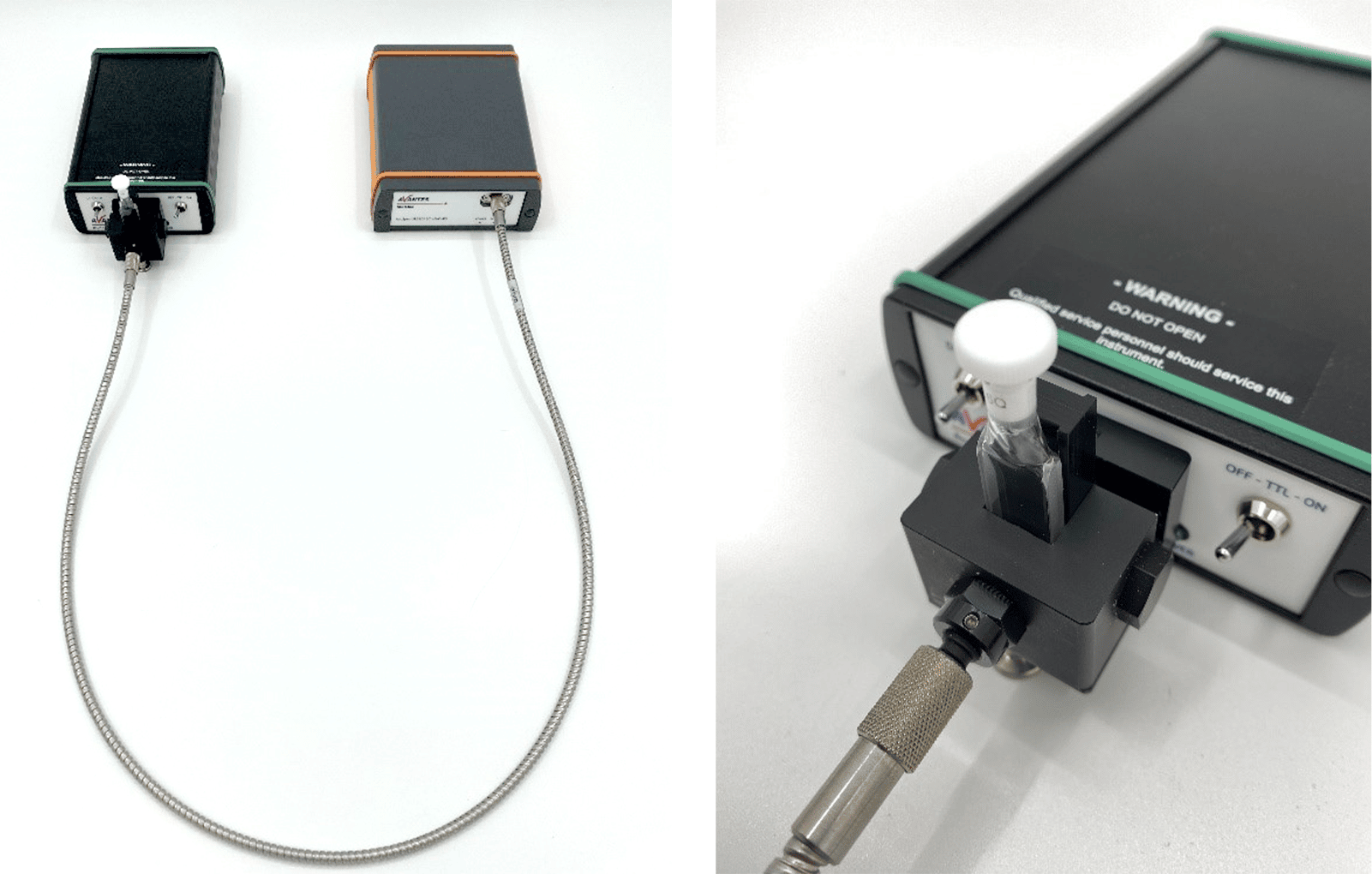
The setup for this experiment (Figure 2) was once again based around the AvaSpec-ULS2048CL-EVO. This device has the latest technology in spectroscopy, which ensures the platform for years to come. This instrument offers USB 3.0 communication, which has ten times the speed of USB2, a CMOS detector, a fast AS-7010 microprocessor, and 50 times more memory capacity, meaning more spectra can be stored onboard and more overall functionality can be realized. Furthermore, this spectrometer can be customized with a wide range of slit sizes, gratings, and fiber optic entrance connectors for a variety of application needs.
The light source used for this experiment was the AvaLight-DHc, which features both a deuterium light source and a halogen light source, giving the user the best of both worlds. The deuterium light provides a UV source between 200-550 nm, and the halogen light provides visible and near-infrared light up to 2500 nm. The AvaLight-DHc also features an internal TTL shutter that is controllable from an AvaSpec spectrometer, and the ability to directly attach a cuvette holder.
Other accessories used for this experiment included a direct attach cuvette holder (CUV-DA) to expose the samples to the maximum amount of light and a 600-micron core fiber optic cable (FC-UVIR600-1-BX) to connect the cuvette holder to the spectrometer. All samples (lemon-lime, blueberry pomegranate, white grape, and black cherry) and the seltzer reference were contained in 2-mm path-length cuvettes. These thinner cuvettes were used to minimize light scattering and better differentiate between samples.
Description of Methodology:
To properly analyze each sparkling water sample, the carbonation in the samples had to be removed, as bubbles can add large amounts of light scattering and therefore distort the results. To accelerate the decarbonation process, the sparkling water samples and the seltzer water reference were poured into capped beakers, which were then shaken multiple times until minimal bubbles appeared with a subsequent shake. The samples and reference were then left exposed to the atmosphere for an additional amount of time to allow any remaining carbonation to dissipate. These samples were measured but showed high levels of saturation with their peaks, meaning the samples had to be diluted. In separate beakers, 100 μm of each sample was mixed with 900 μm of the reference seltzer water. These were the final samples used for analysis. Each sample was pipetted into a 2-mm path-length cuvette to minimize light scattering and further attenuate the saturation of the samples. A spacer was used to keep the thinner cuvettes in place since the holder was designed for 10-mm path-length cuvettes.
For data analysis, we used the absorbance mode in AvaSoft, our exclusive custom software package. This mode is specifically designed for absorbance applications, where the reference measurement will report 0 A.U. (absorbance units) and 5 A.U. when the light source is turned off. In this experiment, the seltzer water was used as the reference to determine the differences between it and the sparkling water samples. We used an integration time of 9 milliseconds, which can be adjusted to increase or decrease the amount of light being measured at one time and affects the overall magnitude of the reported spectrum. We set averaging to 50, meaning 50 values were averaged together to provide more consistent spectra results. This high number for averaging is feasible due to the short integration time.
Test Data and Results:
Displayed below are the absorbance spectra of the samples.
Analysis:
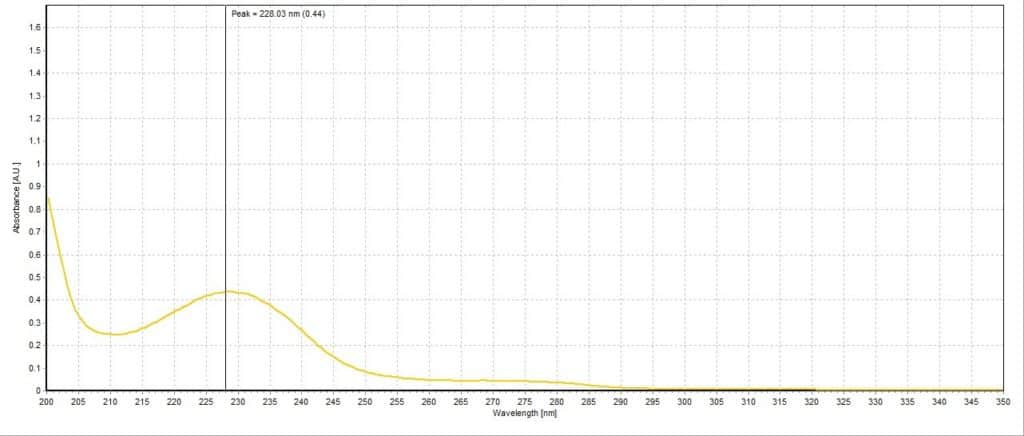
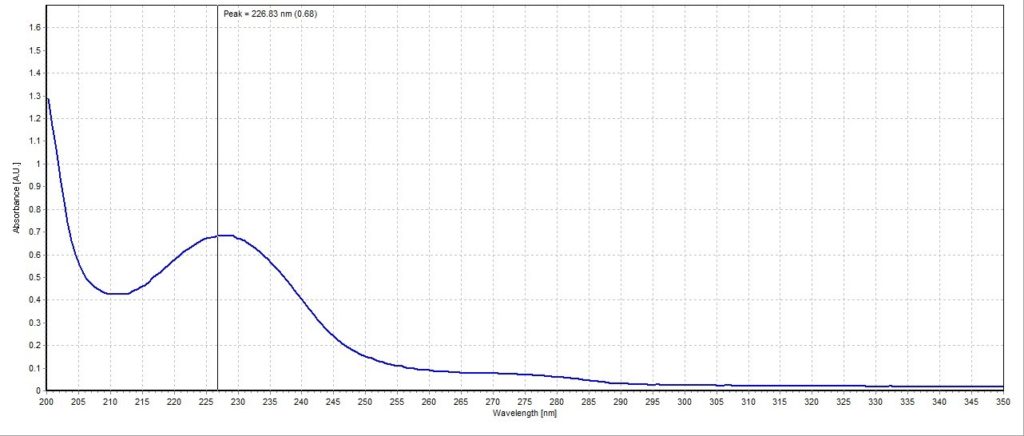
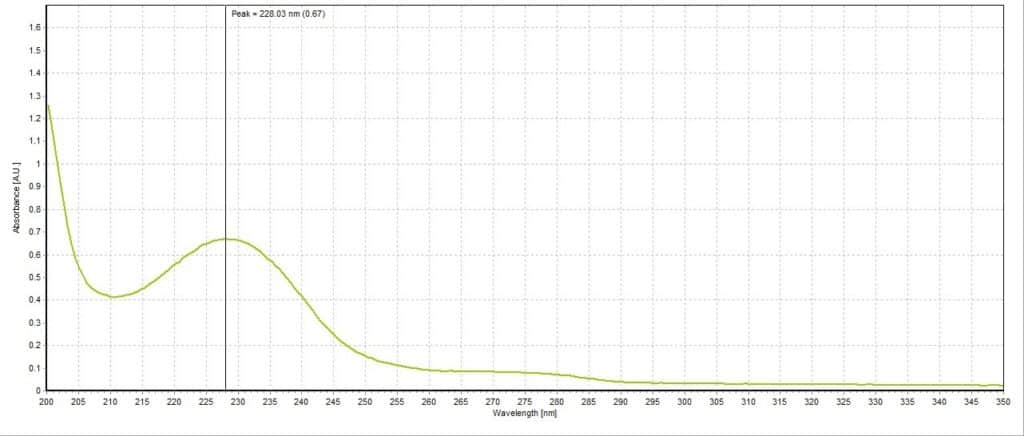
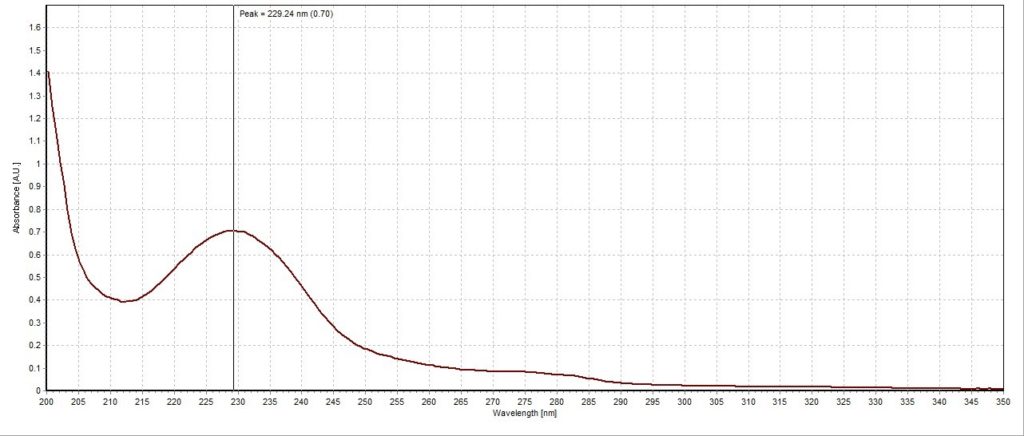
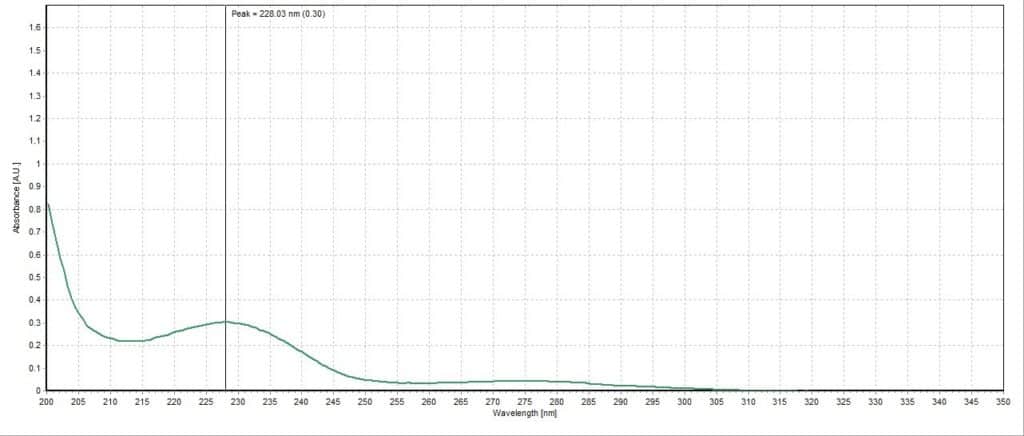
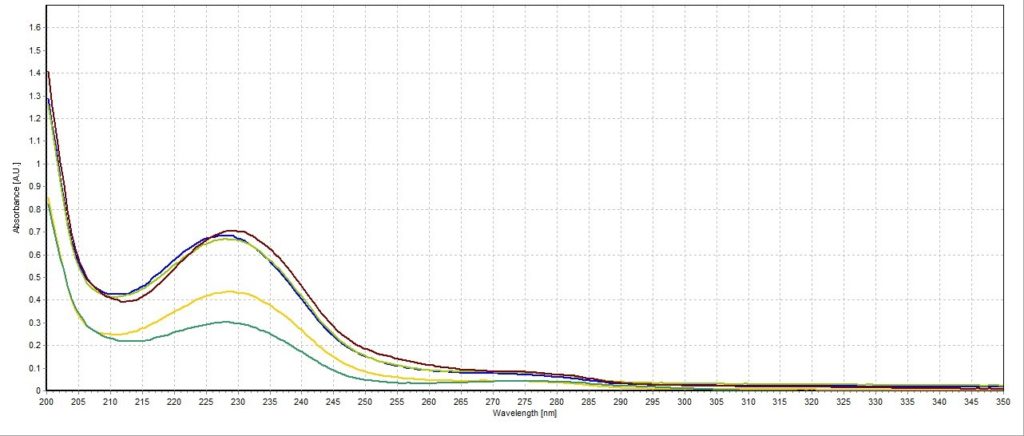
The absorbance spectrum for the lemon lime sample had a measured absorbance peak at 228.03 nm with a magnitude of 0.44 A.U. (Figure 3). The absorbance spectrum for the blueberry pomegranate sample had a measured absorbance peak at 226.83 nm with a magnitude of 0.68 A.U. (Figure 4). The absorbance spectrum for the white grape sample had a measured absorbance peak at 228.03 nm, the same as the lemon lime sample, but with a higher magnitude of 0.67 A.U. (Figure 5). The absorbance spectrum for the black cherry sample had a measured absorbance peak at 229.24 nm with a magnitude of 0.70 A.U. (Figure 6). The absorbance spectrum for the lemon lime soda sample had a measured absorbance peak at 228.03 nm with a magnitude of 0.30 A.U. (Figure 7). A plot of all five spectra is also provided to illustrate the differences in peak location and intensity (Figure 8).
All the sparkling water flavors had similar peak magnitudes around 0.70 A.U. except for lemon lime, which had a substantially lower peak at 0.44 A.U. This may indicate that citrus flavoring is more detectable or distinct to human taste buds compared to the other flavors. Interestingly, the lemon lime flavor was even less present in the lemon lime soda, though this is likely due to the presence of high amounts of sugar and less need for other flavorings. Blueberry pomegranate and black cherry were the two selected flavors that had absorbance peaks furthest apart (226.83 nm and 229.24 nm, respectively). The lemon lime sample, white grape sample, and lemon lime soda sample all reported around the same peak of 228.03 nm. While this is not surprising for the two lemon lime flavored beverages, it is unexpected that the white grape sample would report the same peak. Perhaps studying the molecular composition of the two added flavors may reveal more similarities between the two compared to the other studied flavors.
Conclusion:
In conclusion, the present experiment highlights the small differences that can be observed between different flavored sparkling waters through a comparison of absorbance spectra and illustrates the different amounts of flavoring found in lemon lime sparkling water versus lemon lime soda. The AvaSpec-ULS2048CL-EVO is well suited for a variety of uses, including absorbance, thanks to its versatility and wide range of configurations, and is future-proof for duplicating the experiment for years to come. The AvaLight-DHc is suited for nearly all absorbance applications, with a broad wavelength range from 200 to 2500 nm thanks to its deuterium and halogen bulbs. Please contact Avantes for more information on the configuration that is best suited for your data collection.
 My Cart
My Cart